A Biased View of iHealth COVID-19 Rapid Antigen Home Test - Rhino

9 Simple Techniques For iHealth Statement on the COVID-19 Omicron Variant and Its

Viral loads, which are normally high in early disease, are adequately above the limit of detection for some antigen tests for the first 5 days of signs. After this initial duration, performance decreases quickly as the viral load begins to reduce. We presently have no evidence that versions impact the ability of diagnostic tests approved by Health Canada to validate COVID-19 cases.
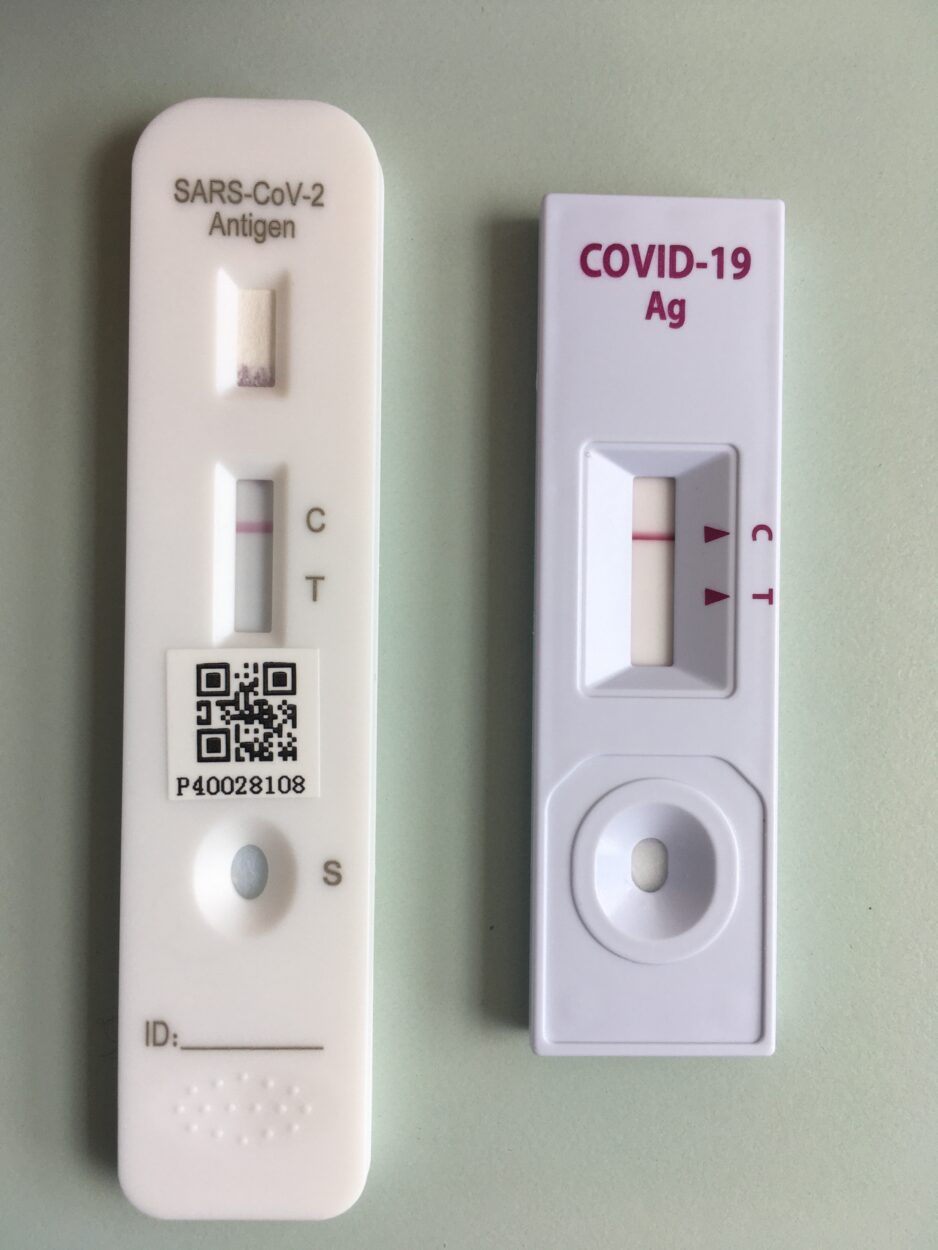
Why home COVID tests remain costly and hard to get in the U.S: Shots - Health News : NPR
If there's a problem, we will act rapidly and keep you informed. Sustainability Health Canada motivates producers to think about the usage of recycled/recyclable, multiple-use, compostable and sustainable products in the development of test devices. For instance, makers might think about making use of recycled/recyclable product packaging products and inserts and/or minimizing one-time usage testing parts for self-tests.
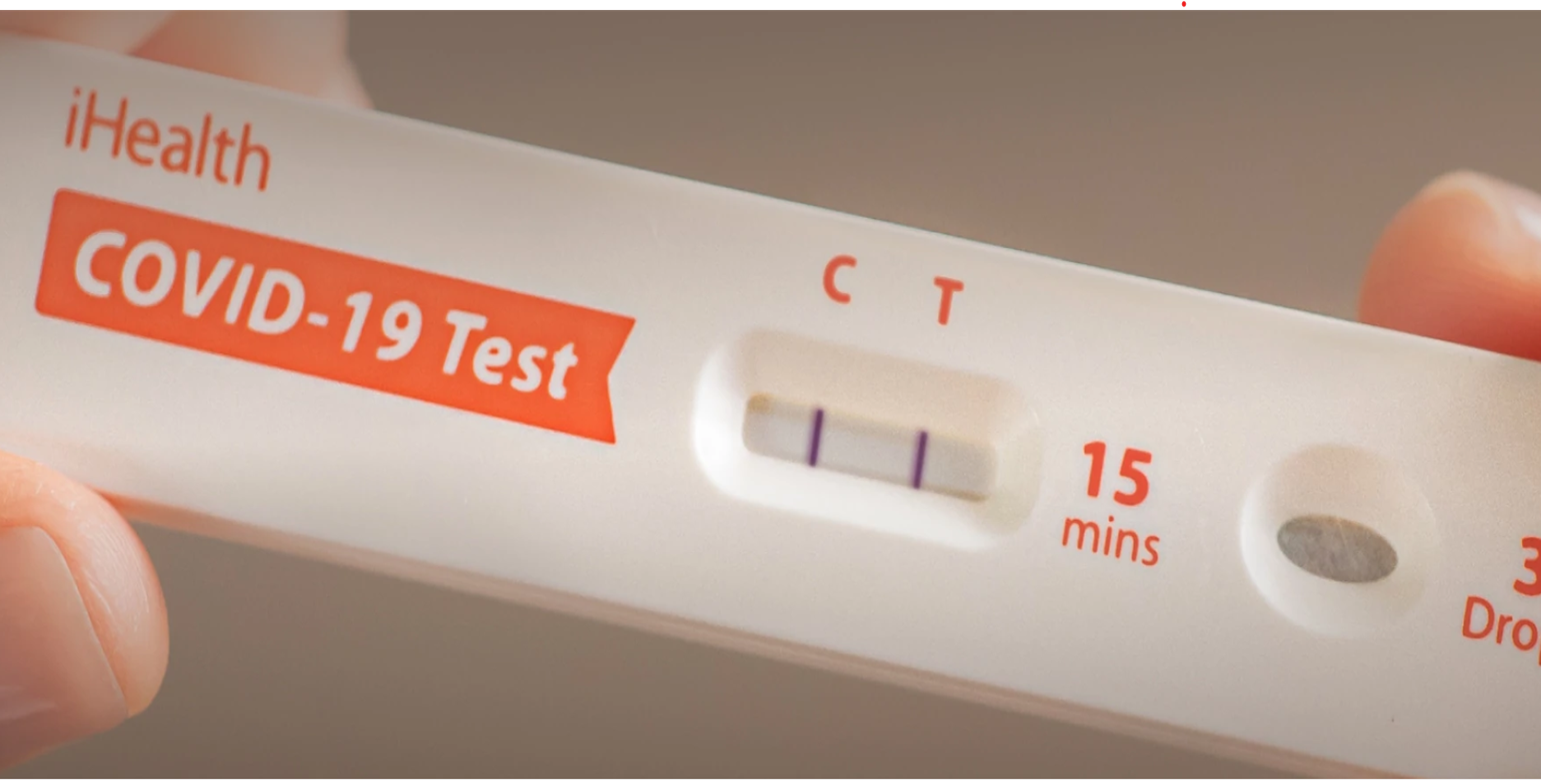
Rapid COVID test kits available in Russell - WWLP
2. Before sending an application, manufacturers are motivated to seek advice from the "Medical devices no longer thought about to have immediate public health need status: Notification to industry" page. The Interim Order No. 2 to import and offer medical gadgets related to COVID-19 enables Health Canada to perform expedited assessments of suitable applications.
About Covid testing: How to get free at-home rapid test kits - ZDNet
As part of the application, producers will be needed to explain the safety, efficiency and quality of their medical gadget. We are waiving the fees for these applications. Health Canada refers to guidance published by the United States Fda (FDA) on. For antigen-based screening devices intended for laboratory or point-of-care use: We have actually likewise set minimum standards for level of sensitivity for a COVID-19 antigen test to fulfill in order for us to consider it for authorization.
For antigen tests meant for self-testing: Manufacturers following the FDA guidance for molecular and antigen tests for non-laboratory use ought to note that Health Canada anticipates them to follow the guidance for. This is because the difference made by the FDA between prescription and non-prescription screening does not exist in Canada.

iHealth OTC Rapid At-Home COVID-19 Antigen Test Kits for Sale - Shop iHealth Antigen Tests
Please note, we have upgraded our policy position on asymptomatic serial testing indications. Speak with https://www.ppesupplyintl.com to learn more on how to apply the new policy to your submission. This notice matches the currently accepted guidance files released by the FDA. Health Canada has extra labelling specifications relating to vaccinations and emerging versions of public health concern that are not consisted of in the FDA assistances.
